Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
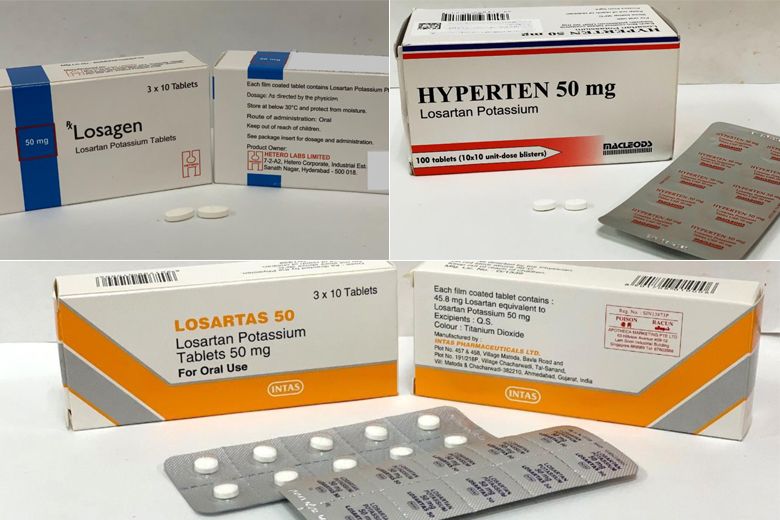
SINGAPORE: About 137,000 patients in Singapore are using the three recalled brands of high blood pressure medicine containing losartan, the Ministry of Health (MOH) said on Thursday (Mar 28).
Earlier, the Health Sciences Authority (HSA) said that the brands Losartas, Losagen and Hyperten were being recalled as the medicines contained higher than acceptable levels of a nitrosamine impurity.
Exposure to nitrosamines at higher quantities over a long-term period may potentially increase the risk of cancer, said HSA.
Seven other brands of losartan medicine approved in Singapore are not affected by the recall.
“About 137,000 patients in Singapore are using the three recalled brands of losartan medicine. Of these, about 130,000 patients have been prescribed Losartas at the public healthcare institutions,” said MOH.
Advertisement
There is no immediate risk associated with taking the affected brands of medicine, according to the ministry.
“Patients are strongly advised to continue taking their medicine until their healthcare providers arrange for them to stop or switch to suitable alternatives.
“Patients who are unsure whether they have been using an affected product should contact their healthcare providers,” said MOH.
CONSULT DOCTOR FOR ALTERNATIVES: MOH
Public healthcare institutions will be reaching out to their patients, MOH said.
Patients whose medical appointments are scheduled before Jul 1 should proceed with these appointments and their doctor will advise them on suitable alternative medicines.
Patients whose medical appointments are scheduled on or after Jul 1 will be contacted for an earlier consultation or medication review, or both.
The public healthcare sector has made additional orders of unaffected brands of losartan, said MOH. The additional supplies will arrive progressively from a few weeks’ time. Importers will also be setting aside additional supplies for private healthcare providers.
“To ensure continued availability of high blood pressure medication for all patients who need them, medical practitioners are advised to initially prescribe the medication on a one-month basis.
“This ensures that patients in all settings are able to promptly receive the medication they need. This may be the practice for the next six months,” MOH said.
At public healthcare institutions, patients currently on losartan will not have to pay more during the interim period should they switch to replacement medicines, which will be priced the same or lower.
Charges incurred for services such as additional consultations or tests to assess suitability for a switch in medicine will also be waived. Refunds will be provided to patients returning the affected losartan medication at their public healthcare institutions, MOH said.
Alternative high blood pressure medication include the Angiotensin Converting Enzyme Inhibitor (ACE-I) class of medicines as well as other angiotensin receptor blocker (ARB) classes of medicines.