Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
WASHINGTON, DC (WICS/WRSP) — A blood pressure medicine that has dealt with at least four recalls this year is facing yet another. This time, the recall is not due to a possible cancer-causing carcinogen.
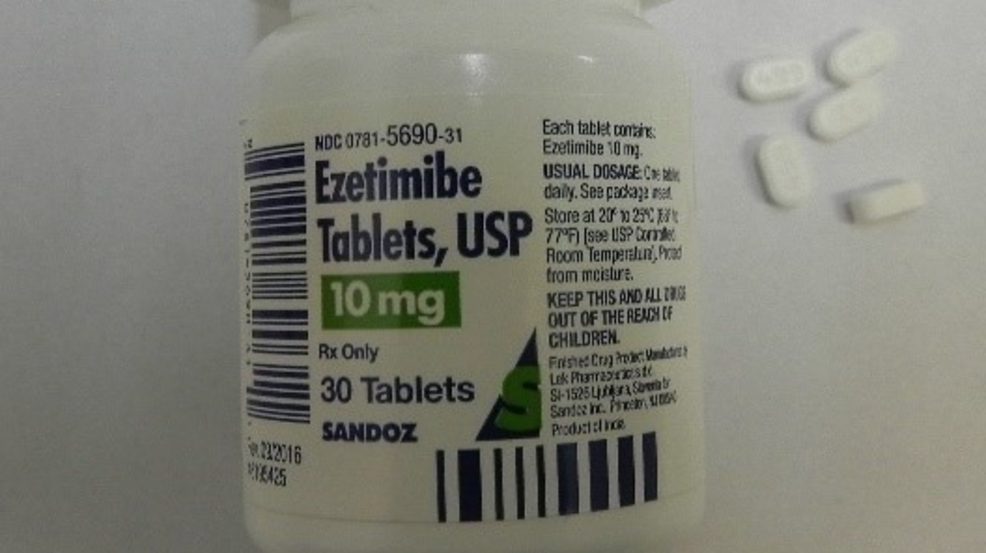
Sandoz Global is recalling 636,000 Losartan Potassium and Ezetimibe prescription drug bottles in U.S.
The U.S. Consumer Product Safety Commission says the bottles fail to meet child-resistant closure requirements, posing a poisoning risk if swallowed by children.
Consumers are advised to immediately secure the medications to keep them out of the sight and reach of children and contact Sandoz for a free replacement child-resistant bottle cap.
The affected bottles were sold at clinics and pharmacies nationwide as a prescribed medicine from July 2018 through August 2019.
So far, no injuries have been reported in relation to this recall.
For more information contact 800-525-8747 or visit www.us.sandoz.com.
The full recall notice listing the bottles affected can be found at http://bit.ly/32afA0s
Source: https://newschannel20.com/news/nation-world/blood-pressure-medicine-recalled-for-poisoning-risk