Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
A year after Torrent Pharmaceuticals first began recalling tainted losartan products manufactured at its Indrad plant, the FDA paid a visit. It found a plant that often routinely reported products out of specification but frequently shipped them to the U.S. anyway.
While the liberal redactions in a 17-page Form 483 generated from that visit make it impossible to know whether Torrent’s losartan products were among those that were a problem, the findings indicate a plant with issues.
An investigation found that in a two-year period ending in March of this year, 340 finished product batches tested out of spec. But in 73% of those cases, the plant retested then reversed those findings, often on grounds the FDA said were baseless. The retests were then reported for the record. With stability testing, it happened 61% of the time.
The inspectors found that the plant did not properly calibrate equipment and that it had no written procedures in place to make sure drugs met identity, strength and quality standards.
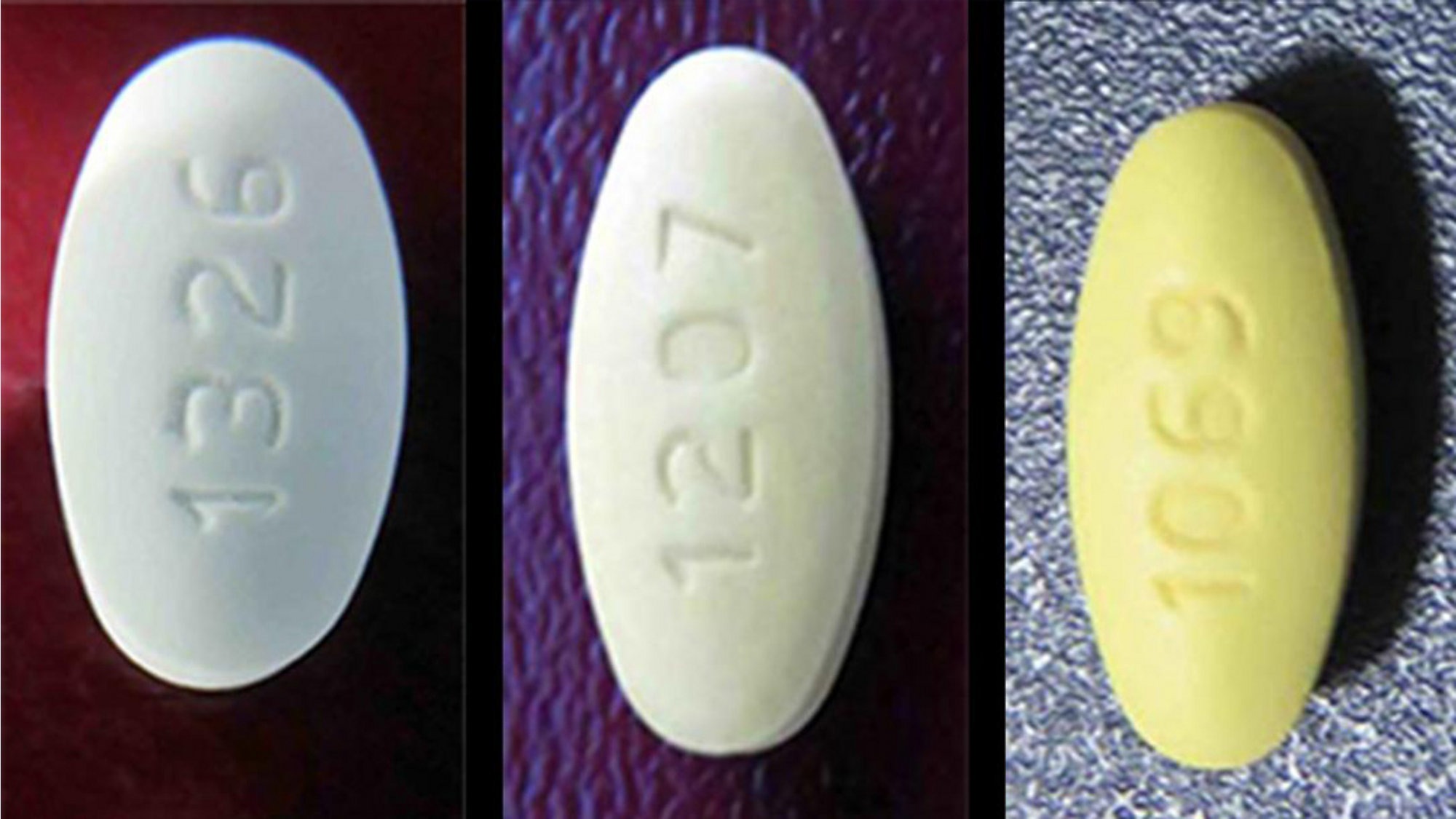
Torrent is just one of the companies that has been in the midst of the FDA’s discovery of blood pressure drugs that were found to have been made with APIs whose manufacturing process generated impurities. Those impurities are considered probable cancer-causing agents. An API produced in India by Hetero Labs figured into the most recent recall. The discoveries have led to a massive recall, and in some cases shortages, of losartan, valsartan and irbesartan products.
While other drugmakers like Sandoz and Teva have also been recalling products, the numbers from Torrent have been enormous. There have been 17 reported events, according to an FDA website, that have resulted in the recall of more than 2.1 million bottles of losartan potassium tablets and losartan potassium/hydrochlorothiazide tablets in a variety of dose strengths.
Source: https://www.fiercepharma.com/manufacturing/fda-finds-testing-issues-at-torrent-plant