Over 10 years experience of Traceability Solutions
By pharmatrax
Category: News
 No Comments
No Comments
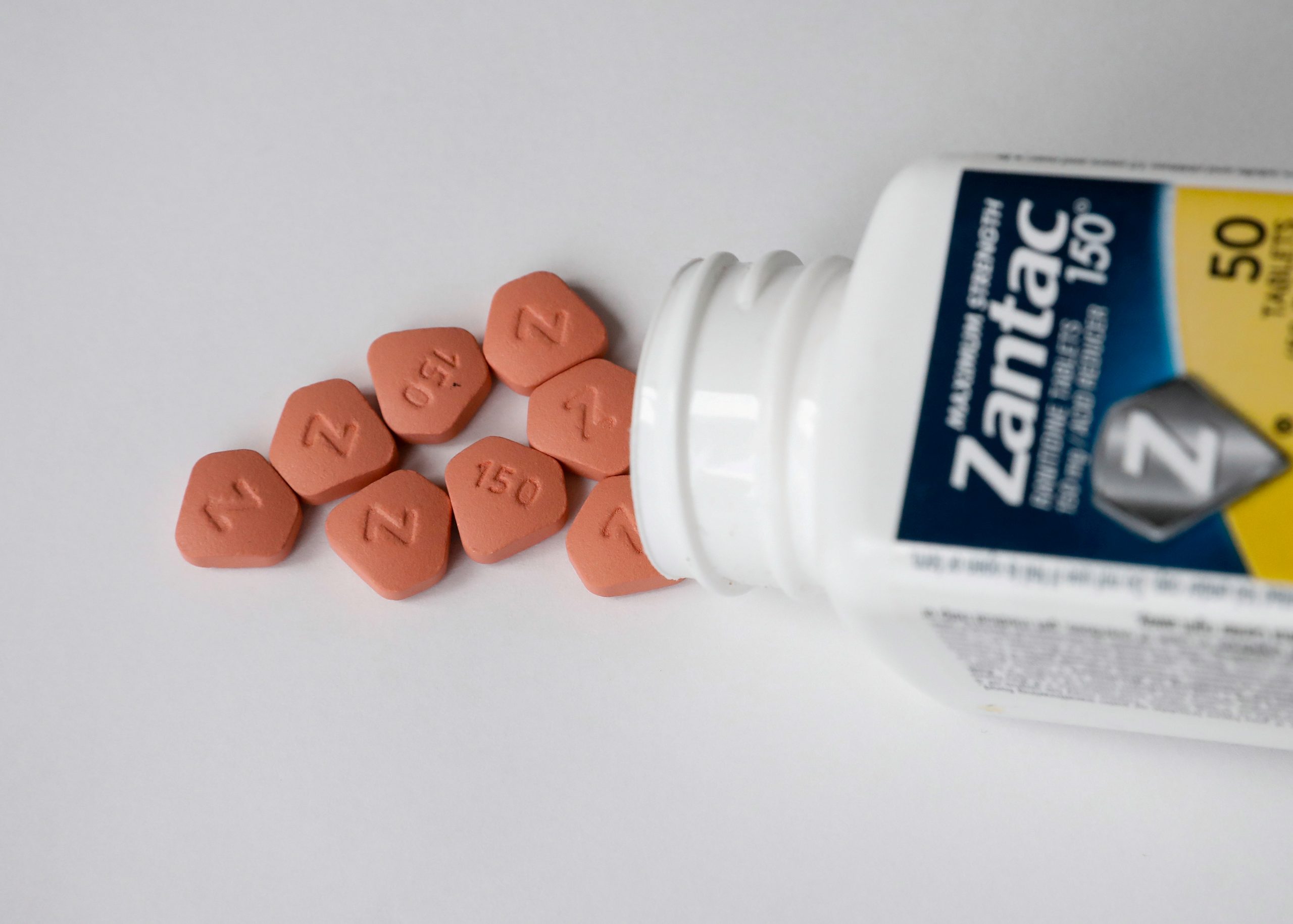
BAXTER SPRINGS, Ks. — The FDA is recalling some common heartburn medications because of the presence of an impurity that may cause cancer.
Denton Pharma Inc. recalled several batches of antacid medications that may contain a carcinogenic ingredient.
The FDA says the ingredient, NDMA, is found in ranitidine tablets, Zantac, and other heartburn medications.
The recall impacts all unexpired lots of 150 milligram and 300-milligram tablets.
NDMA is becoming an increasing concern in medications.
Neal Schmidt, Pharmacist, Wolkar Drug, said, “There are people calling and asking whether they should be concerned. We check cause we get updates on recalls, so we check our medication, check the lot, or the batch and that type of thing to see if it’s a recalled lot or batch.
The most recent FDA recall last fall reported trace amounts of the contaminant had been found in both branded and generic versions of the drug, triggering weeks of recalls.