Over 10 years experience of Traceability Solutions

By Pharmatrax Author
Category: Technoloy
 No Comments
No Comments
Finding a cure for cancer has been called this century’s “moonshot.” As a senior scientist at Molecular Devices, I know first-hand that accelerating cancer drug discovery is a priority of not only our research teams, but also of our private and public customers around the globe. To achieve the goal of finding a cure, we will need enormous research and development resourcing, focused attention, and extraordinary collaboration. We’ll also need better tools.

For more than a century, two-dimensional (2D) cell cultures have been the standard for researchers and scientists trying to understand disease and explore potential cures. However, 2D cell cultures have some inherent flaws. They aren’t representative of real cell environments. They lack predictivity. They have issues caused by the growth media and expansion of cells. Members of the scientific community have been experiencing a quiet technological revolution in cell imaging that is allowing them to view cells like never before: in 3D.
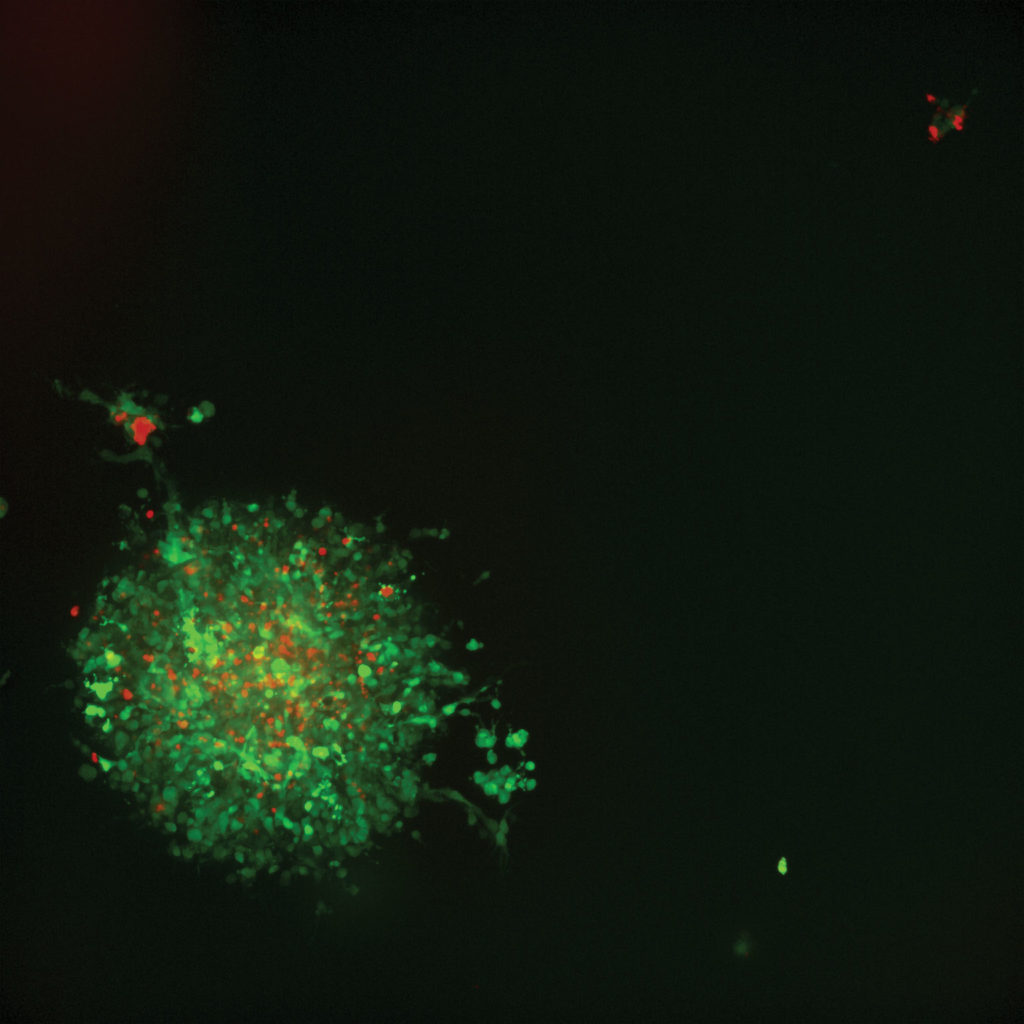
In drug discovery, the closer laboratory-prepared cell cultures mimic human cells in vivo, the better scientists and researchers can understand how various compounds will affect them in the real world. Cancer drug development with 3D imaging relies on groupings of cells called spheroids. Cancer spheroids are believed to mimic tumor behavior far more effectively than standard 2D cell cultures because, much like tumors, they contain both surface-exposed and deeply buried cells, proliferating and nonproliferating regions, and a hypoxic center with a well-oxygenated outer layer of cells. Such 3D spheroid models are being successfully used in screening environments for identifying potential cancer therapeutics.
Already there have been many advancements because of 3D imaging technology, and I believe we are just at the beginning.
In a laboratory setting, the preparation of protocols, assays, and other materials are necessary but time-consuming steps. But several recent cancer drug studies using 3D imaging devices have had a timeline of 7–10 days from initialization to final image processing, with spheroids formed and ready for anticancer compound treatments in as little as 24 hours.
An added benefit of the speed with which researchers can prepare an experiment is the ability to note errors in process, failed compound treatments, or other clues that mean the experiment should be discarded. Failing fast is more than a catchphrase. With 3D imaging, we can accelerate cancer drug development by knowing what isn’t working and quickly moving to the next idea.
With many anticancer compounds under research and in trials, data showing how they affect cancer cells must be extremely accurate. The ability of in vitro 3D culture systems to produce uniform human cancer cell spheroids, coupled with the ability to screen spheroid response at high speeds and high volume, signify a major step in facilitating more relevant and accurate testing. For example, in a recent study that measured effects of various anticancer compounds, we found that larger spheroids were more resistant to paclitaxel cytotoxicity, a fact that would have been unavailable to scientists using 2D cultures.
Further, the now-possible ability for researchers to count individual cancer cells within a spheroid for cell death—a measure of the efficacy of a potential drug—means the difference between whether a drug might work, and how well it works relative to others. Counting and scoring of individuals cells is necessary for accurately understanding cancer cell death and rating various anticancer drugs.
Our research with confocal 3D image analysis of cancer cells has shown the ability to do multiparametric image analysis and quantify different biological outputs. In one analysis, effects were characterized using several compounds representing six different classes of anticancer drugs. From this work, we were able to statistically characterize various phenotypic changes in the spheroids in response to drug treatment—a substantial leap in potential drug efficacy.
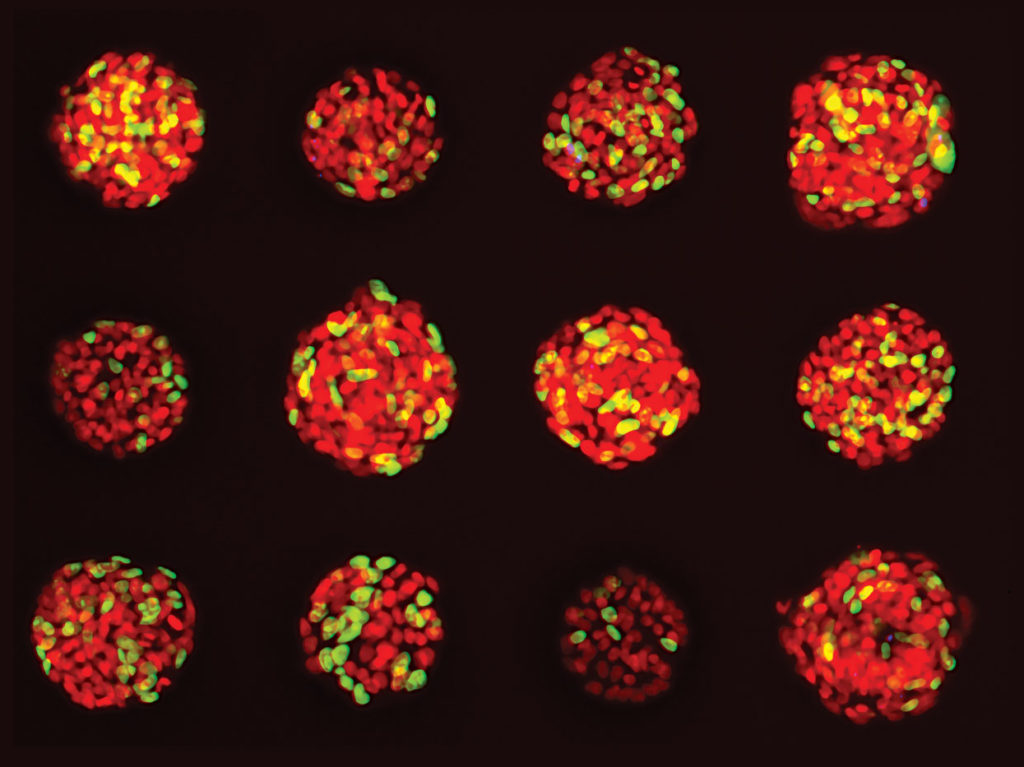
Being able to count and view multiple parameters with 3D imaging means a better understanding of how a specific anticancer compound is affecting a specific spheroid—an ultra-individualized look at potential drug performance. For example, in one study our team performed, researchers observed significant changes after compound treatment. There was an overall reduction in the number of spheroids, and we observed a concentration-dependent decrease in the sum volume of live cells within each spheroid, the spheroid size, and the number of viable cells.
In other words, 3D allowed us to view the effects of a drug on a population of cancer spheroids all the way down to individual cells within a single spheroid, and from there extrapolate specific dosing recommendations for anticancer compounds. More and more individualized data means better drug development processes and ultimately better outcomes for patients.
Personalized medicine is another hot topic in cancer drug discovery that is expedited and improved with 3D imaging. There is growing interest in developing methods for this arena, in which tumor cells from individual patients are tested for sensitivity to a panel of drugs to prescribe the most effective treatment, saving the patient the discomfort—or worse—of experiencing the process-of-elimination approach in an attempt to find the right treatment. As individualized testing becomes more common, many doctors and laboratories will start demanding more relevant, time-sensitive studies. This is possibly the most impactful and patient-centric avenue resulting from widespread 3D imaging use.
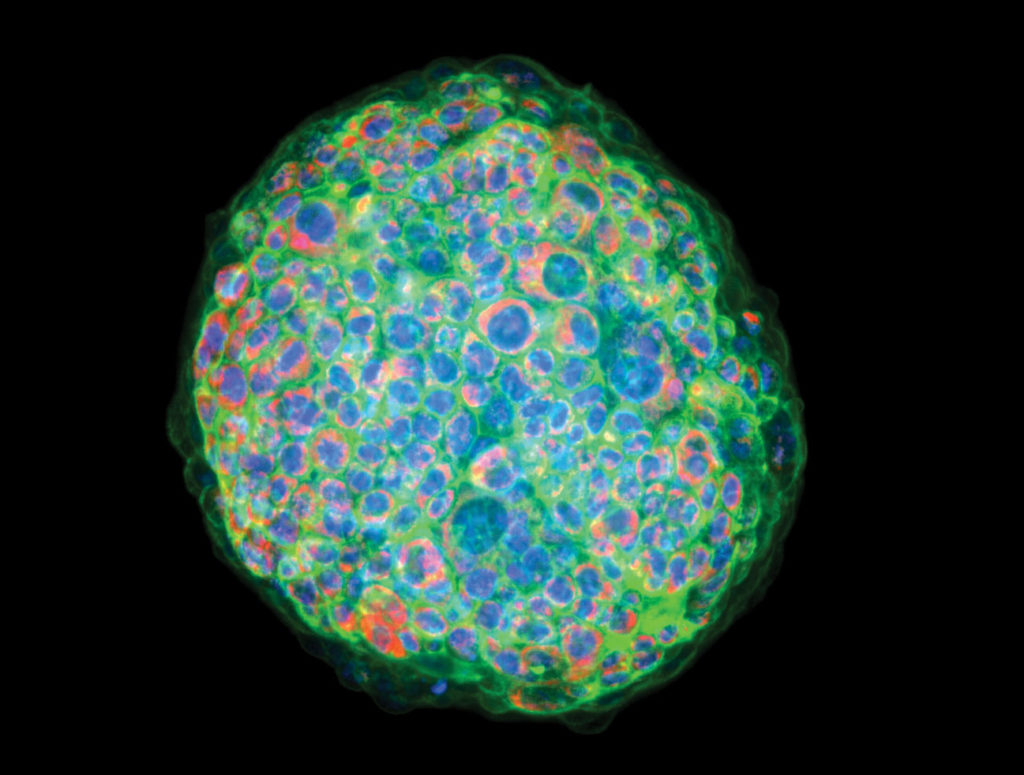
What is next in cancer drug exploration? I believe we are only seeing the tip of the iceberg. There is so much yet to uncover, so much unknown. What I do know is that it’s clear that 3D imaging is part of a new generation of technologies enabling us to speed discovery with incredible accuracy and individualization.